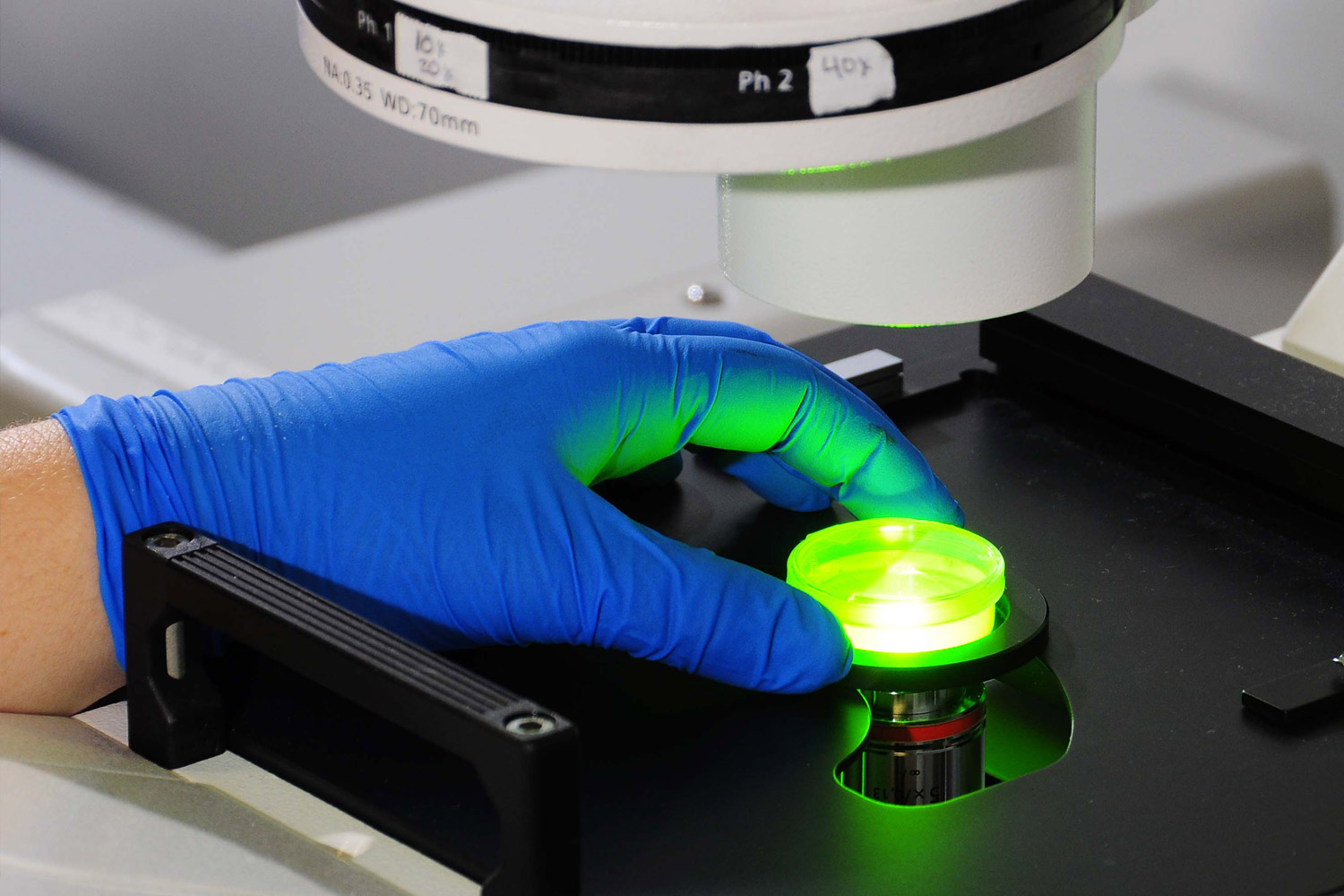
The goal, said Bryan Brown, executive director of BioWV, is to “really provide easy to understand and succinct breakdowns of what is in the pipeline currently for vaccines to combat COVID, which ones have the best chance of making it out and generally what kind of timeframe are we looking at for when those would be widely available.”
Following Arthur, Marsh will discuss in-depth the state’s plans for implementation, which starts with target populations like first responders, healthcare professionals, seniors and those with chronic diseases, said Brown.
As outlined by the DHHR’s COVID-19 Vaccination Plan, the plan involves three stages of implementation based on availability once a vaccine is approved: Once high-risk populations have received vaccines and supply is available, the DHHR anticipates more accessibility to West Virginia residents through a “broader network” of immunization providers. The third phase will shift the COVID-19 vaccination to become a “routine” immunization.
Brown said he hopes the forum will provide more clarity for fellow West Virginians as the pandemic continues.
“A safe and effective vaccine will help bring life back to normal,” Brown said. “We’re all in this shared experience of COVID-19, and we all want to see this go away. This forum will work to shed light on where we are in the process of developing vaccines and treatments for COVID-19. It’s an issue that impacts every West Virginian, and we hope to relay and convey this message with every West Virginian in an easy to understand one-stop shop fashion.”
He also hopes the efforts to provide transparency about the industry’s developments dispels distrust or skepticism surrounding vaccination trial and approval processes.
“There’s an extremely rigorous process by which the U.S. Food and Drug Administration goes about monitoring, evaluating and managing the clinical trial process through which the different vaccine candidates go through,” Brown said. “The [FDA] has pledged to be open and transparent about every aspect of this clinical trial process, the approval process for the vaccines.”
As companies aggressively search for a vaccine for COVID-19, Brown said he’s noticed an unprecedented level of collaboration occurring throughout the industry.
“I don’t think that there has ever been a more robust effort on a single issue than the effort that has been placed on finding a cure for COVID-19 on behalf of the global biopharmaceutical industry and global research community,” Brown said. “It’s an incredible effort to watch from afar, and ultimately we’re going to have to trust the science and scientists that come up with the vaccine that is approved if we want to combat and defeat COVID-19.”
For more information about the event or to register for the webinar, visit https://www.biowv.org/events.html. Event sponsors include Mylan, VWR, BIO, PhRMA, Pfizer, Novartis and Advantage Technologies.